Research in our lab is dedicated to understanding the role of the Alzheimer's disease risk factor Apolipoprotein E (APOE) in cerebral metabolism. With a special focus on glia and their contributions to neurodegeneration, we employ a variety of transcriptomic and metabolomic approaches (from cells to mice to humans) in order to identify early-life changes in metabolism associated with APOE. Our lab's overarching goal is to discover new therapeutic targets that will allow us to reprogram metabolism in individuals at-risk for Alzheimer's Disease in order to enhance cognitive resilience and to extend the brain’s health span.
Ongoing Projects
|
APOE & the PPP
The central hypothesis of this project is that APOE influences neuronal function and survival through isoform-specific changes in glucose metabolism via the pentose phosphate pathway (PPP). To test our hypothesis, we are employing a unique ‘omics approach to test a novel mechanism which potentially ties E4 to neuropathological changes in cerebral glucose metabolism, oxidative stress and neuronal survival. |
Mapping Metabolism in the Aging Brain
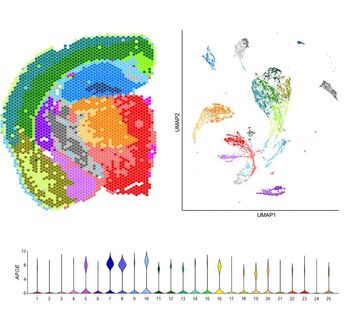
Cerebral metabolism is based on a complex interplay between multiple cell types. What types of changes do each of these cells undergo during aging and in disease? Does APOE play a role? To begin to try to answer these questions, we are using a combination of single cell and spatial transcriptomic approaches to map metabolic changes in the brain across multiple cell types and brain regions. [In collaboration with Morganti and Sun labs] |
Lipid Droplets, APOE, & AD
Interestingly, one of Dr. Alois Alzheimer's original findings in 1907 was that glial cells contained "adipose saccules." Many now think he was referring to lipid droplets. Does APOE have a role to play in modulating lipid droplet dynamics in the Alzheimer's disease (AD) brain? Our lab is attempting to answer that question using a combination of in vitro, in vivo and ex vivo approaches to probe the role of APOE in modulating lipid metabolism and lipid droplet biology. |
The APOE "Switch" Mouse:
A Novel Mouse Model Our novel transgenic model, the APOE “switch mouse” (4s2), uses the Cre-loxP system to allow for inducible APOE allele switching from APOE4 to APOE2. Our exciting preliminary studies leverage this new mouse as a promising model to assess the therapeutic potential of replacing APOE4 with APOE2 and to investigate how we can utilize ApoE2’s neuroprotective effects to mitigate ApoE4-associated AD pathologies. Ongoing studies aim to determine whether cell-specific replacement of APOE4 with APOE2 will rescue E4-associated metabolic dysfunction, disease-associated gene signatures, and AD pathology.
|
Our lab is supported by generous funding from the following: